Projects
Filter by:
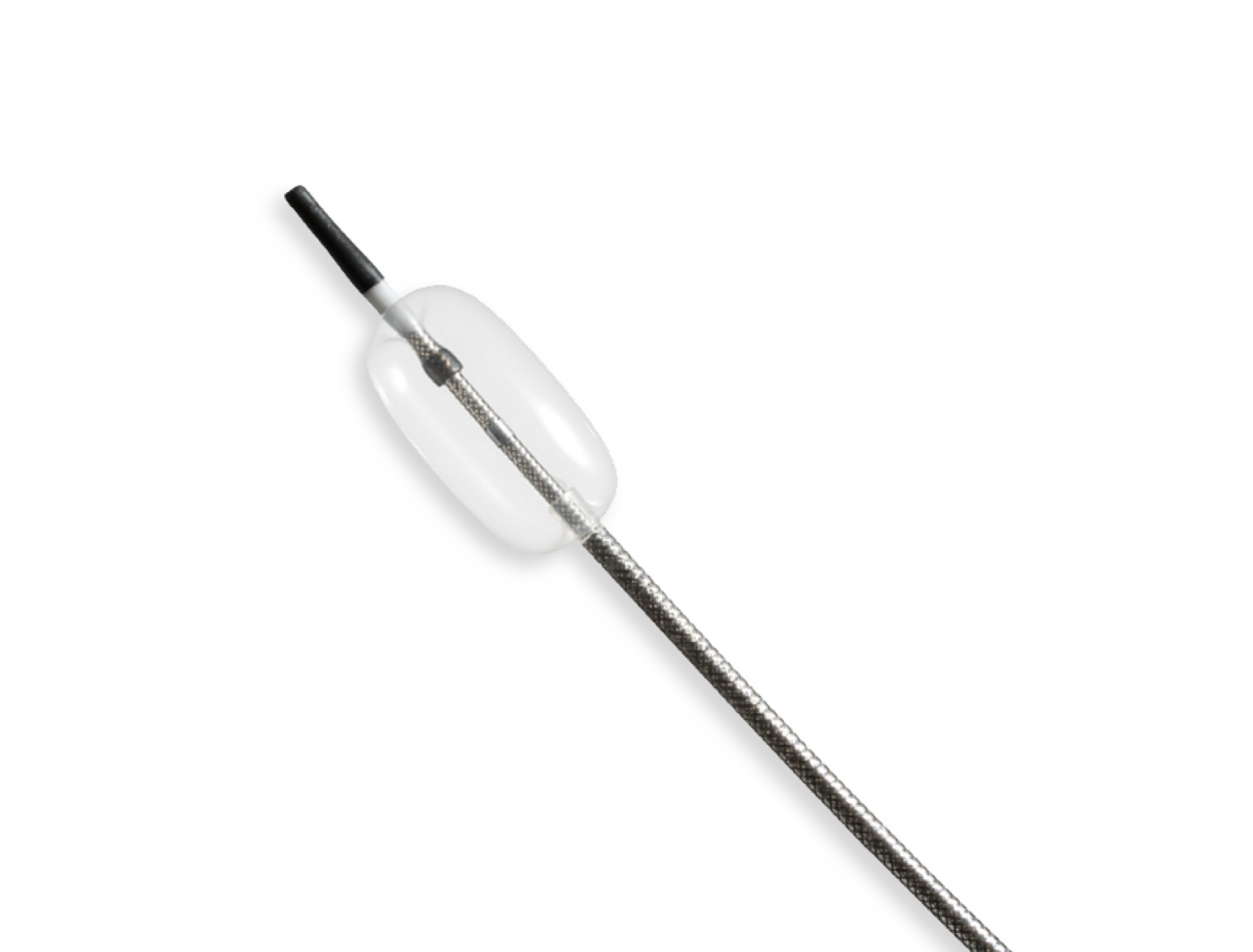
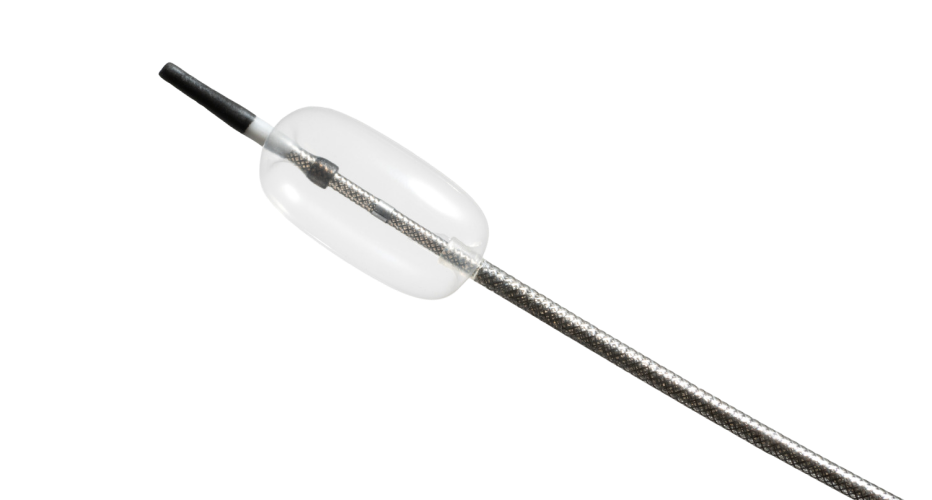
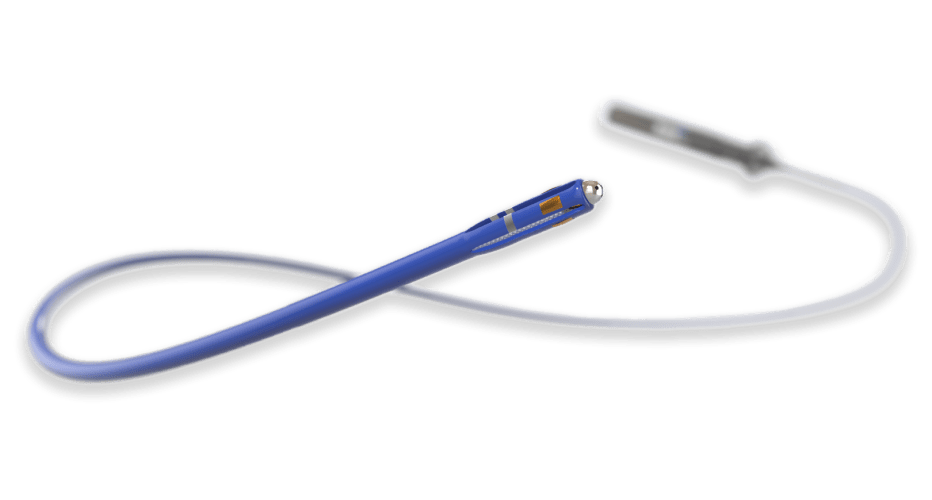
Compliant Balloon Microcatheter
In collaboration with the customer’s R&D engineers, we developed a highly specialized microcatheter using a compliant occlusion balloon.
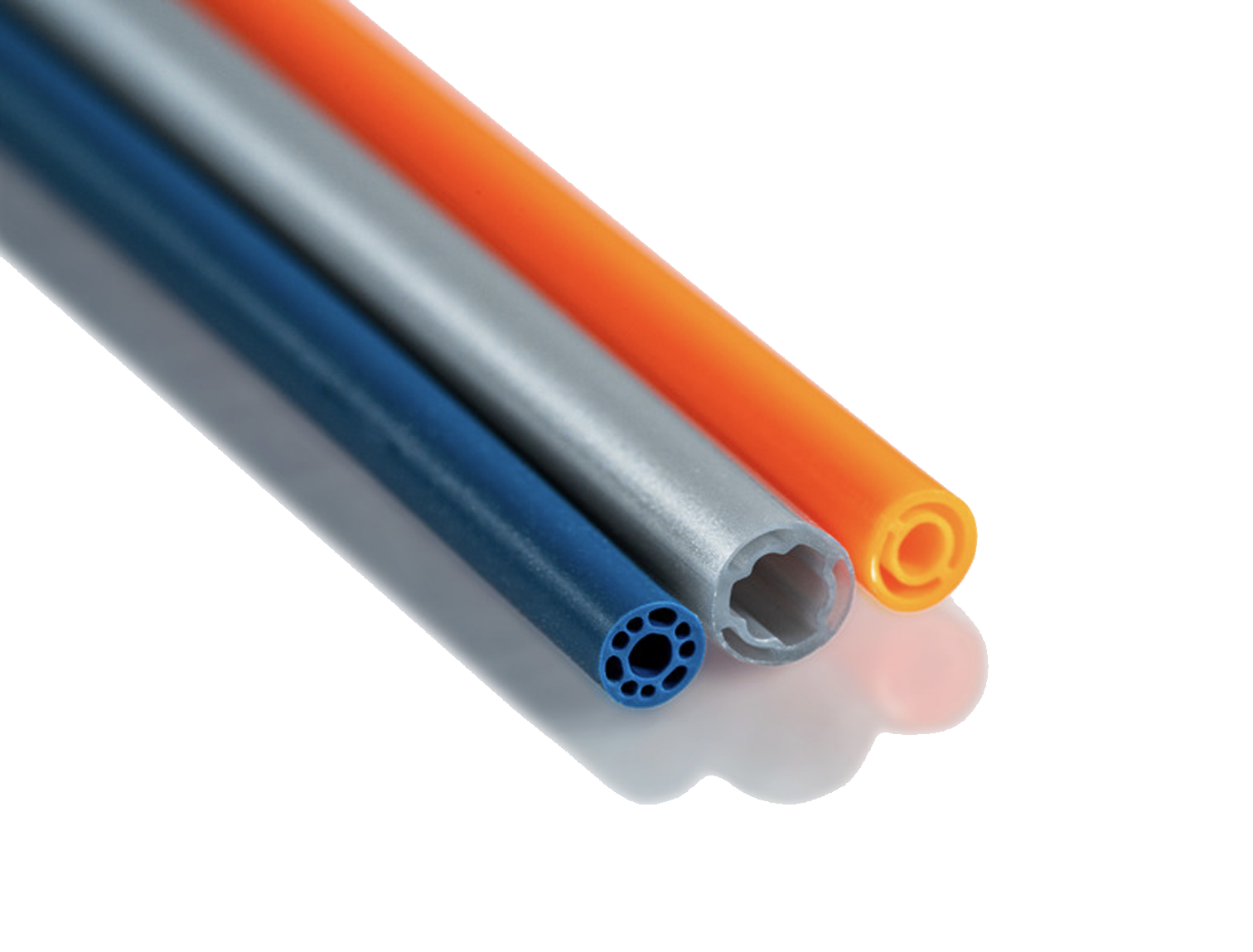
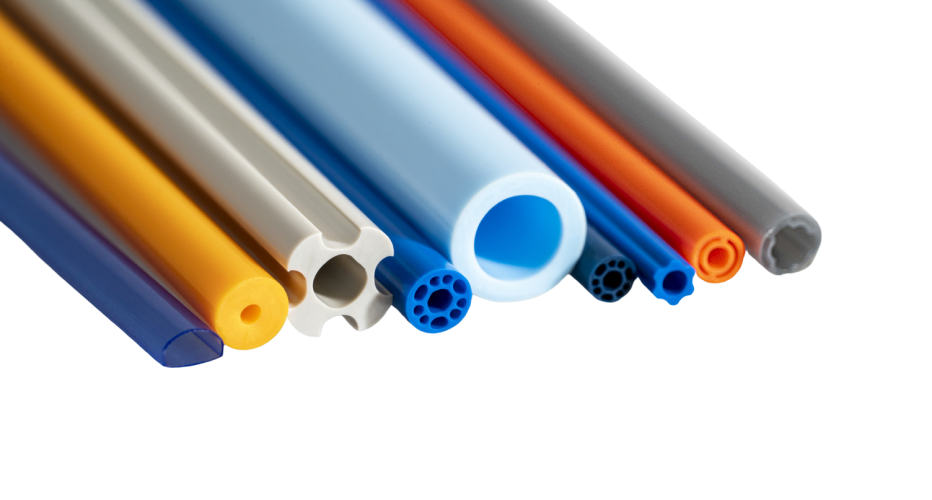
To meet exacting requirements, a custom co-axial braided catheter shaft was designed with exceptional pushability, lubricity, and kink resistance.
The unique design featuring a sub-3Fr catheter profile, flexible body, atraumatic tip, and compliant balloon allows for maximum patient compatibility.
Compliant balloon materials often require advanced bonding techniques to create a robust, leak-proof bond to the catheter body. Typically, this bonding creates an increased profile across the bond area. However, for this project to meet clinical requirements, the device needed a smooth, homogenous profile.
In close collaboration with the customer’s R&D engineers, we developed a highly customized balloon bonding process. A specialized reinforced braided catheter shaft was engineered enabling a device design with exceptional pushability, lubriciousness, and kink resistance.
We improved the customer’s development schedule while maintaining all design requirements. Yields of 98%+ were achieved through rigorous leak testing.
“A genuine and highly collaborative partner, and a natural extension of our R&D and production team.”
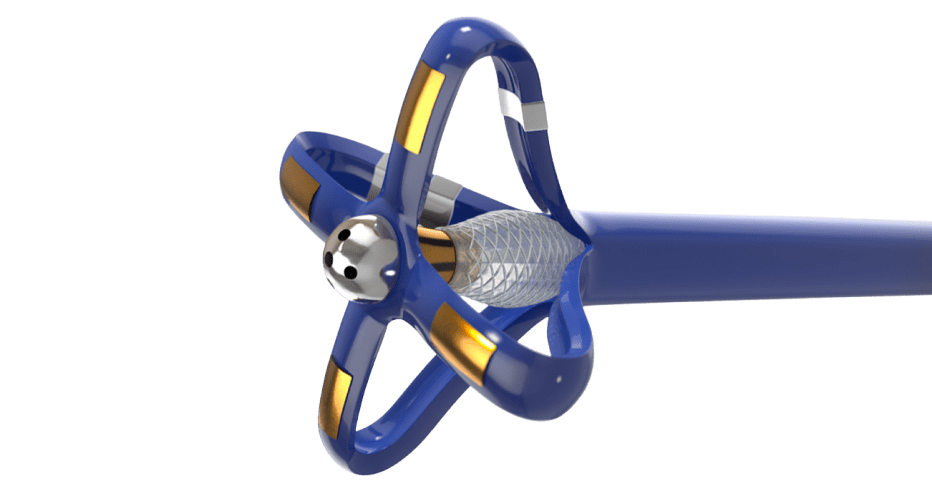
Non-Compliant Balloon Catheter
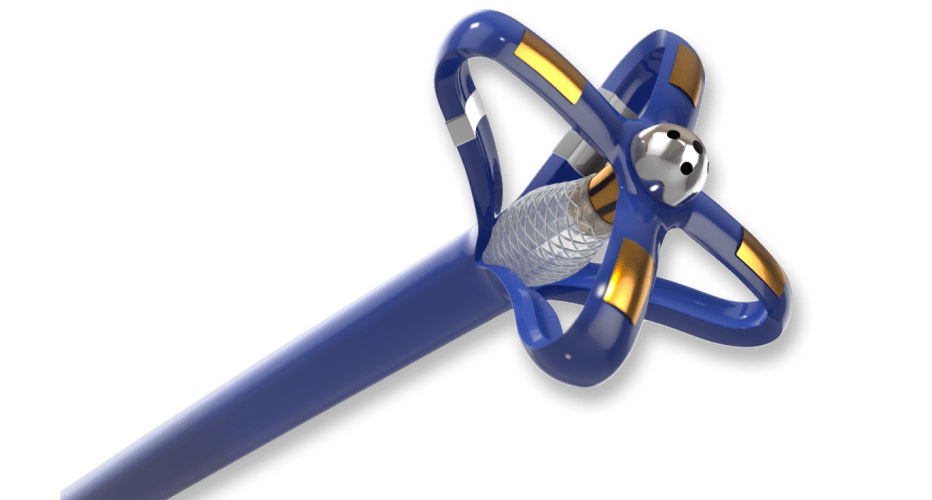
We designed, developed, and manufactured a non-compliant balloon catheter with specially placed markers to allow the physician unparalleled visualization before deploying the stent.
Balloon catheters typically incorporate inflation and deflation lumens to operate. These additional lumens can negatively impact catheter device flexibility because of the complex internal geometry. To precisely place the stent, the cardiologist requires flexible balloon catheters along with clear visualization under fluoroscopy.
We partnered with the customer to develop a novel and “industry-first” catheter reinforcement method. The resulting solution allowed the inflation and deflation lumens to function without impacting the catheter’s flexibility.
Our close collaboration with the customer across all stages of the program development led to the device completing pre-clinical trials in record time. It is now in validation before entering clinical trials for human use.
“Outstanding in their ability to help us develop an optimal manufacturing process. Always listening to our needs and responding to meet milestones, they are a genuine partner.”